We probably use electricity everyday for many different purposes
but do we really know what electricity is?
This is a very complicated question, and as you dig deeper and ask more questions, there really is not a definitive answer, only abstract representations of how electricity interacts with our surroundings.......
Electricity is briefly defined as the flow of electric charge, but there’s so much behind that simple statement. Where do the charges come from? How do we move them? Where do they move to? How does an electric charge cause mechanical motion or make things light up? So many questions! To begin to explain what electricity is we need to zoom way in, beyond the matter and molecules, to the atoms that make up everything we interact with in life.
To understand the fundamentals of electricity, we need to begin by focusing in on atoms, one of the basic building blocks of life and matter. Atoms exist in over a hundred different forms as chemical elements like hydrogen, carbon, oxygen, and copper. Atoms of many types can combine to make molecules, which build the matter we can physically see and touch.
Let's look at the Ant on a leaf.....
We think of it that the Ant is very small but the atom is very small...Much much smaller..
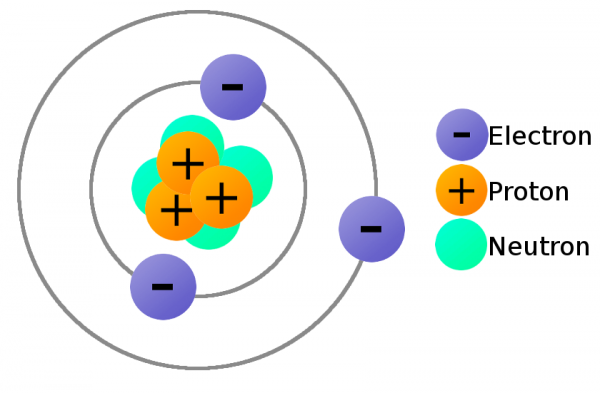
In atomic physics, the Rutherford–Bohr model or Bohr model or Bohr diagram, introduced by Niels Bohr and Ernest Rutherford in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons (Most of the space is taken up by the area where the electrons exist) that travel in circular orbits around the nucleus—similar in structure to the Solar System, but with attraction provided by electrostatic forces rather than gravity.
Story behind Rutherford_Bohr Atomic Model
Rutherford Scattering
Ernest Rutherford said one day “hey, I think I will shoot some stuff at atoms.” I am sure his wife said “oh, Ernie” (she probably called him Ernie) “if it makes you happy to play with your little physics stuff, go ahead. I know how much you like it.” So he did. He shot some alpha particles (which are really just the nucleus of a helium atom) at some really thin gold foil. Here is a diagram of his experiment.
If you shoot these positive alpha particles at this positive pudding atom, they should mostly bounce off, right? Well, that is not what happened. Rutherford found that most of them went right through the foil. Some of them did bounce back. How could that be if the plumb pudding model was correct? Rutherford’s experiment prompted a change in the atomic model. If the positive alpha particles mostly passed through the foil, but some bounced back. AND if they already knew that the electron was small and negative, then the atom must have a small positive nucleus with the electrons around them.
Bohr Model
The model proposed by Niels Bohr is the one that you will see in a lot of introductory science texts. There are a lot of good ideas in this model, but it is not the one that agrees with all of the current evidence. The model tries to make a connection between light and atoms.
Suppose you take some light and you let different colors bend different amounts (think rainbow). This way, you could see what colors are present for different light sources. Here are three different light sources.
Maybe the light from the light bulb is what you would expect. These are the colors of the rainbow. However, suppose you took some hydrogen gas and excited it. There would only be certain colors (only certain wavelengths) of light produced. If you shine light through some hydrogen gas, there will be dark bands of light at those same colors.
So, Bohr said that these colors of light in the hydrogen gas correspond to different energy levels the electron in hydrogen can have. And this is the key to the Bohr model – electrons can ONLY be at certain energy levels in the atom. This is crazy (at least it was crazy for its time). Think about a planet orbiting the Sun. It can be at any energy level. In this case, there is a gravitational force attracting the planet which produces orbital motion. This will work anywhere in the solar system.
Early physicist thought of the electron in an atom a lot like a planet orbiting the Sun. The key difference is that the electron (in the Bohr model) orbits due to an electric interaction and not a gravitational interaction. Well, the other difference in the Bohr model is that the electron can not orbit (if it does orbit, which it doesn’t) at any distance and any energy. Here is the essence of the Bohr model.
The Bohr model depends on a connection between the frequency of light and the energy of the level change. If light of a frequency corresponding to the energy change interacts with the atom, the electron can absorb the light and jump up a level. If an excited electron jumps down a level, it looses energy. The energy the electron loses becomes light with a frequency corresponding to a the change in energy.
The Bohr model can be quite confusing to introductory students, but the important point is that this model agrees with the following evidence.
- Electrons are small and negatively charged
- Protons are in the nucleus with is small compared to the size of the atom
- For a particular element, only certain frequencies (colors) of light are absorbed or emitted.
Schrodinger and Heisenberg Model
There is a key point about the Bohr model that is no longer accepted in current models of the atom. In the Bohr model, the electrons are still thought to orbit the nucleus just like planets orbit the sun. Actually, this is something that we can not say is true. The problem with atoms and electrons is that we humans except them to obey the same rules as things like baseballs and planets. Actually, the rules are the same, but baseballs and planets follow the rules of quantum mechanics without us humans even noticing.
It turns out that we can’t really say anything about the trajectory or position of electrons in an atom. What we can say is all about probabilities. We can say what regions an electron is likely to be.
Now coming back to electricity....
As we mentioned at the beginning, electricity is defined as the flow of electric charge. Charge is a property of matter–just like mass, volume, or density. It is measurable. Just as we can quantify how much mass something has, we can measure how much charge it has. The key concept with charge is that it can come in two types: positive (+) or negative (-).
In order to move charge we need charge carriers, and that’s where our knowledge of atomic particles–specifically electrons and protons–comes in handy. Electrons always carry a negative charge, while protons are always positively charged. Neutrons (true to their name) are neutral, they have no charge. Both electrons and protons carry the same amount of charge, just a different type.
A lithium atom (3 protons) model with the charges labeled.
The charge of electrons and protons is important, because it provides us the means to exert a force on them. Electrostatic force!
Electrostatic Force
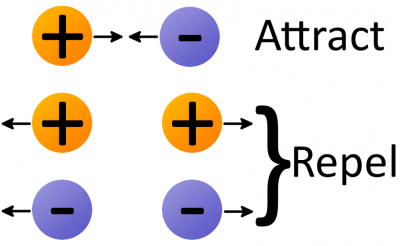
Electrostatic force (also called Coulomb’s law) is a force that operates between charges. It states that charges of the same type repel each other, while charges of opposite types are attracted together. Opposites attract, and likes repel.
The amount of force acting on two charges depends on how far they are from each other. The closer two charges get, the greater the force (either pushing together, or pulling away) becomes.
Thanks to electrostatic force, electrons will push away other electrons and be attracted to protons. This force is part of the “glue” that holds atoms together, but it’s also the tool we need to make electrons (and charges) flow!
Making Charges Flow
We now have all the tools to make charges flow. Electrons in atoms can act as our charge carrier, because every electron carries a negative charge. If we can free an electron from an atom and force it to move, we can create electricity.
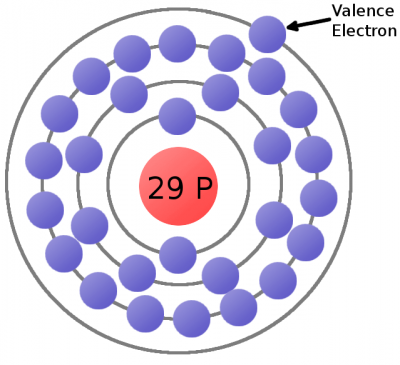
Consider the atomic model of a copper atom, one of the preferred elemental sources for charge flow. In its balanced state, copper has 29 protons in its nucleus and an equal number of electrons orbiting around it. Electrons orbit at varying distances from the nucleus of the atom. Electrons closer to the nucleus feel a much stronger attraction to the center than those in distant orbits. The outermost electrons of an atom are called the valence electrons, these require the least amount of force to be freed from an atom.
This is a copper atom diagram: 29 protons in the nucleus, surrounded by bands of circling electrons. Electrons closer to the nucleus are hard to remove while the valence (outer ring) electron requires relatively little energy to be ejected from the atom.
Using enough electrostatic force on the valence electron–either pushing it with another negative charge or attracting it with a positive charge–we can eject the electron from orbit around the atom creating a free electron.
Now consider a copper wire: matter filled with countless copper atoms. As our free electron is floating in a space between atoms, it’s pulled and prodded by surrounding charges in that space. In this chaos the free electron eventually finds a new atom to latch on to; in doing so, the negative charge of that electron ejects another valence electron from the atom. Now a new electron is drifting through free space looking to do the same thing. This chain effect can continue on and on to create a flow of electrons called electric current.
A very simplified model of charges flowing through atoms to make current.
Conductivity
Some elemental types of atoms are better than others at releasing their electrons. To get the best possible electron flow we want to use atoms which don’t hold very tightly to their valence electrons. An element’s conductivity measures how tightly bound an electron is to an atom.
Elements with high conductivity, which have very mobile electrons, are called conductors. These are the types of materials we want to use to make wires and other components which aid in electron flow. Metals like copper, silver, and gold are usually our top choices for good conductors.
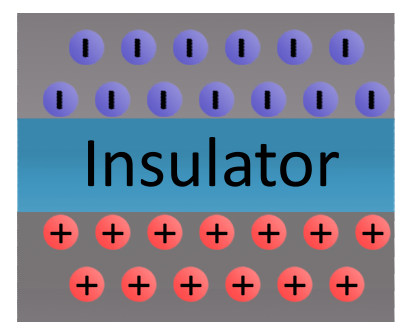
Elements with low conductivity are called insulators. Insulators serve a very important purpose: they prevent the flow of electrons. Popular insulators include glass, rubber, plastic, and air.
Static or Current Electricity
Before we get much further, let’s discuss the two forms electricity can take: static or current. In working with electronics, current electricity will be much more common, but static electricity is important to understand as well.
Static Electricity
Static electricity exists when there is a build-up of opposite charges on objects separated by an insulator. Static (as in “at rest”) electricity exists until the two groups of opposite charges can find a path between each other to balance the system out.
When the charges do find a means of equalizing, a static discharge occurs. The attraction of the charges becomes so great that they can flow through even the best of insulators (air, glass, plastic, rubber, etc.).
Static discharges can be harmful depending on what medium the charges travel through and to what surfaces the charges are transferring. Charges equalizing through an air gap can result in a visible shock as the traveling electrons collide with electrons in the air, which become excited and release energy in the form of light.
Spark gap igniters are used to create a controlled static discharge. Opposite charges build up on each of the conductors until their attraction is so great charges can flow through the air.
One of the most dramatic examples of static discharge is lightning. When a cloud system gathers enough charge relative to either another group of clouds or the earth’s ground, the charges will try to equalize. As the cloud discharges, massive quantities of positive (or sometimes negative) charges run through the air from ground to cloud causing the visible effect we’re all familiar with.
Static electricity also familiarly exists when we rub balloons on our head to make our hair stand up, or when we shuffle on the floor with fuzzy slippers and shock the family cat (accidentally, of course). In each case, friction from rubbing different types of materials transfers electrons. The object losing electrons becomes positively charged, while the object gaining electrons becomes negatively charged. The two objects become attracted to each other until they can find a way to equalize.
Working with electronics, we generally don’t have to deal with static electricity. When we do, we’re usually trying to protect our sensitive electronic components from being subjected to a static discharge. Preventative measures against static electricity include wearing ESD (electrostatic discharge) wrist straps, or adding special components in circuits to protect against very high spikes of charge.
Current Electricity
Current electricity is the form of electricity which makes all of our electronic gizmos possible. This form of electricity exists when charges are able to constantly flow. As opposed to static electricity where charges gather and remain at rest, current electricity is dynamic, charges are always on the move. We’ll be focusing on this form of electricity throughout the rest of the tutorial.
Circuits
In order to flow, current electricity requires a circuit: a closed, never-ending loop of conductive material. A circuit could be as simple as a conductive wire connected end-to-end, but useful circuits usually contain a mix of wire and other components which control the flow of electricity. The only rule when it comes to making circuits is they can’t have any insulating gaps in them.
If you have a wire full of copper atoms and want to induce a flow of electrons through it, all free electrons need somewhere to flow in the same general direction. Copper is a great conductor, perfect for making charges flow. If a circuit of copper wire is broken, the charges can’t flow through the air, which will also prevent any of the charges toward the middle from going anywhere.
On the other hand, if the wire were connected end-to-end, the electrons all have a neighboring atom and can all flow in the same general direction.
We now understand how electrons can flow, but how do we get them flowing in the first place? Then, once the electrons are flowing, how do they produce the energy required to illuminate light bulbs or spin motors? For that, we need to understand electric fields.
Electric Fields
We have a handle on how electrons flow through matter to create electricity. That’s all there is to electricity. Well, almost all. Now we need a source to induce the flow of electrons. Most often that source of electron flow will come from an electric field.
What’s a Field?
A field is a tool we use to model physical interactions which don’t involve any observable contact. Fields can’t be seen as they don’t have a physical appearance, but the effect they have is very real.
We’re all subconsciously familiar with one field in particular: Earth’s gravitational field, the effect of a massive body attracting other bodies. Earth’s gravitational field can be modeled with a set of vectors all pointing into the center of the planet; regardless of where you are on the surface, you’ll feel the force pushing you towards it.
The strength or intensity of fields isn’t uniform at all points in the field. The further you are from the source of the field the less effect the field has. The magnitude of Earth’s gravitational field decreases as you get further away from the center of the planet.
As we go on to explore electric fields in particular remember how Earth’s gravitational field works, both fields share many similarities. Gravitational fields exert a force on objects of mass, and electric fields exert a force on objects of charge.
Electric Fields
Electric fields (e-fields) are an important tool in understanding how electricity begins and continues to flow. Electric fields describe the pulling or pushing force in a space between charges. Compared to Earth’s gravitational field, electric fields have one major difference: while Earth’s field generally only attracts other objects of mass (since everything is so significantly less massive), electric fields push charges away just as often as the attract them.
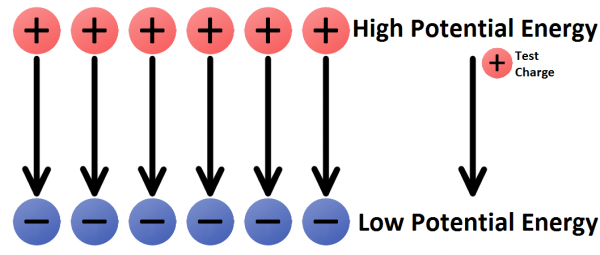
The direction of electric fields is always defined as the direction a positive test charge would move if it was dropped in the field. The test charge has to be infinitely small, to keep its charge from influencing the field.
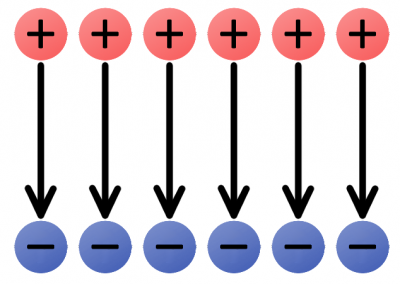
We can begin by constructing electric fields for solitary positive and negative charges. If you dropped a positive test charge near a negative charge, the test charge would be attracted towards the negative charge. So, for a single, negative charge we draw our electric field arrows pointing inward at all directions. That same test charge dropped near another positive charge would result in an outward repulsion, which means we draw arrows going out of the positive charge.
The electric fields of single charges. A negative charge has an inward electric field because it attracts positive charges. The positive charge has an outward electric field, pushing away like charges.
Groups of electric charges can be combined to make more complete electric fields.
The uniform e-field above points away from the positive charges, towards the negatives. Imagine a tiny positive test charge dropped in the e-field; it should follow the direction of the arrows. As we’ve seen, electricity usually involves the flow of electrons–negative charges–which flow against electric fields.
Electric fields provide us with the pushing force we need to induce current flow. An electric field in a circuit is like an electron pump: a large source of negative charges that can propel electrons, which will flow through the circuit towards the positive lump of charges.
Electric Potential (Energy)
When we harness electricity to power our circuits, gizmos, and gadgets, we’re really transforming energy. Electronic circuits must be able to store energy and transfer it to other forms like heat, light, or motion. The stored energy of a circuit is called electric potential energy.
Energy? Potential Energy?
To understand potential energy we need to understand energy in general. Energy is defined as the ability of an object to do work on another object, which means moving that object some distance. Energy comes in many forms, some we can see (like mechanical) and others we can’t (like chemical or electrical). Regardless of what form it’s in, energy exists in one of two states: kinetic or potential.
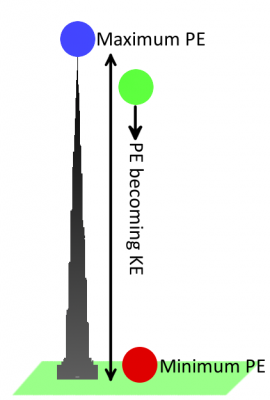
An object has kinetic energy when it’s in motion. The amount of kinetic energy an object has depends on its mass and speed. Potential energy, on the other hand, is a stored energy when an object is at rest. It describes how much work the object could do if set into motion. It’s an energy we can generally control. When an object is set into motion, its potential energy transforms into kinetic energy.
Let’s go back to using gravity as an example. A bowling ball sitting motionless at the top of Khalifa tower has a lot of potential (stored) energy. Once dropped, the ball–pulled by the gravitational field–accelerates towards the ground. As the ball accelerates, potential energy is converted into kinetic energy (the energy from motion). Eventually all of the ball’s energy is converted from potential to kinetic, and then passed on to whatever it hits. When the ball is on the ground, it has a very low potential energy.
Electric Potential Energy
Just like mass in a gravitational field has gravitational potential energy, charges in an electric field have an electric potential energy. A charge’s electric potential energy describes how much stored energy it has, when set into motion by an electrostatic force, that energy can become kinetic, and the charge can do work.
Like a bowling ball sitting at the top of a tower, a positive charge in close proximity to another positive charge has a high potential energy; left free to move, the charge would be repelled away from the like charge. A positive test charge placed near a negative charge would have low potential energy, analogous to the bowling ball on the ground.
To instill anything with potential energy, we have to do work by moving it over a distance. In the case of the bowling ball, the work comes from carrying it up 163 floors, against the field of gravity. Similarly, work must be done to push a positive charge against the arrows of an electric field (either towards another positive charge, or away from a negative charge). The further up the field the charge goes, the more work you have to do. Likewise, if you try to pull a negative charge away from a positive charge–against an electric field–you have to do work.
For any charge located in an electric field its electric potential energy depends on the type (positive or negative), amount of charge, and its position in the field. Electric potential energy is measured in units of joules (J).
Electric Potential
Electric potential builds upon electric potential energy to help define how much energy is stored in electric fields. It’s another concept which helps us model the behavior of electric fields. Electric potential is not the same thing as electric potential energy!
At any point in an electric field the electric potential is the amount of electric potential energy divided by the amount of charge at that point. It takes the charge quantity out of the equation and leaves us with an idea of how much potential energy specific areas of the electric field may provide. Electric potential comes in units of joules per coulomb (J/C), which we define as a volt (V).
In any electric field there are two points of electric potential that are of significant interest to us. There’s a point of high potential, where a positive charge would have the highest possible potential energy, and there’s a point of low potential, where a charge would have the lowest possible potential energy.
One of the most common terms we discuss in evaluating electricity is voltage. A voltage is the difference in potential between two points in an electric field. Voltage gives us an idea of just how much pushing force an electric field has.
With potential and potential energy under our belt we have all of the ingredients necessary to make current electricity. Let’s do it!
Electricity in Action!
After studying particle physics, field theory, and potential energy, we now know enough to make electricity flow. Let’s make a circuit!
First we will review the ingredients we need to make electricity:
- The definition of electricity is the flow of charge. Usually our charges will be carried by free-flowing electrons.
- Negatively-charged electrons are loosely held to atoms of conductive materials. With a little push we can free electrons from atoms and get them to flow in a generally uniform direction.
- A closed circuit of conductive material provides a path for electrons to continuously flow.
- The charges are propelled by an electric field. We need a source of electric potential (voltage), which pushes electrons from a point of low potential energy to higher potential energy.
A Short Circuit
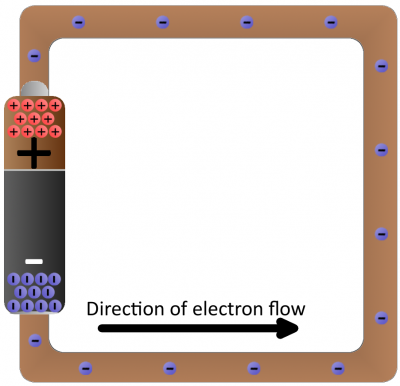
Batteries are common energy sources which convert chemical energy to electrical energy. They have two terminals, which connect to the rest of the circuit. On one terminal there are an excess of negative charges, while all of the positive charges coalesce on the other. This is an electric potential difference just waiting to act!
If we connected our wire full of conductive copper atoms to the battery, that electric field will influence the negatively-charged free electrons in the copper atoms. Simultaneously pushed by the negative terminal and pulled by the positive terminal, the electrons in the copper will move from atom to atom creating the flow of charge we know as electricity.
After a second of the current flow, the electrons have actually moved very little–fractions of a centimeter. However, the energy produced by the current flow is huge, especially since there’s nothing in this circuit to slow down the flow or consume the energy. Connecting a pure conductor directly across an energy source is a bad idea. Energy moves very quickly through the system and is transformed into heat in the wire, which may quickly turn into melting wire or fire.
Illuminating a Light Bulb
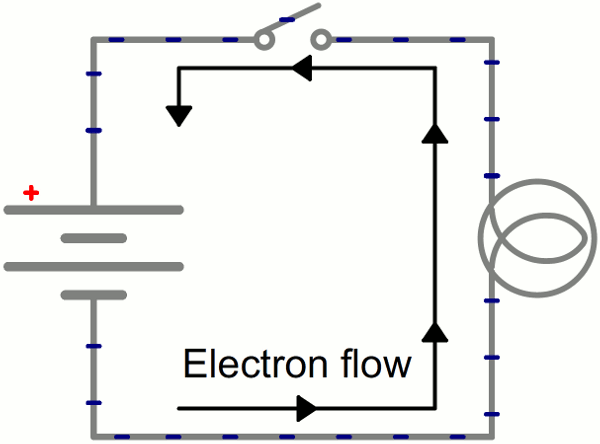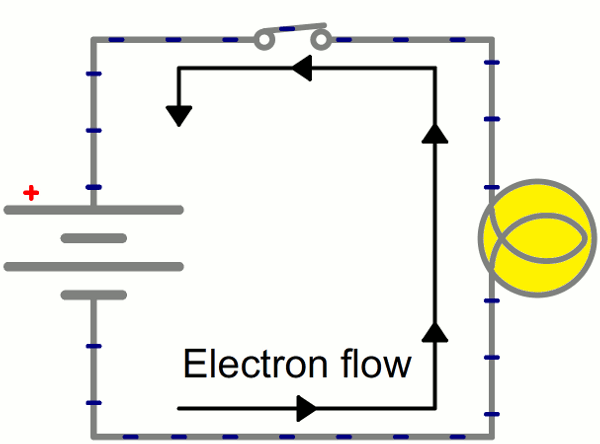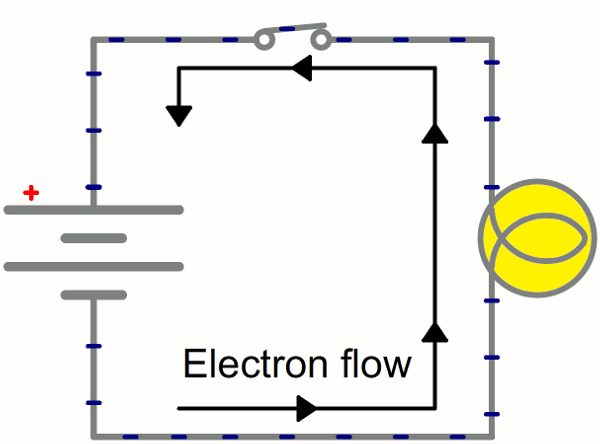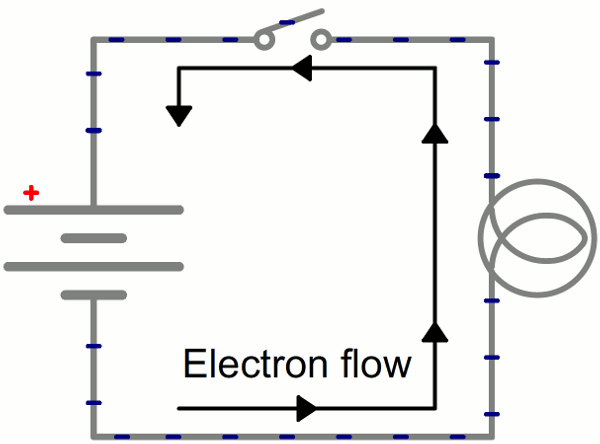
Instead of wasting all that energy, not to mention destroying the battery and wire, let’s build a circuit that does something useful! Generally an electric circuit will transfer electric energy into some other form–light, heat, motion, etc. If we connect a light bulb to the battery with wires in between, we have a simple, functional circuit.
Schematic: A battery (left) connecting to a lightbulb (right), the circuit is completed when the switch (top) closes. With the circuit closed, electrons can flow, pushed from the negative terminal of the battery through the lightbulb, to the positive terminal.
While the electrons move at a snails pace, the electric field affects the entire circuit almost instantly (we’re talking speed of light fast). Electrons throughout the circuit, whether at the lowest potential, highest potential, or right next to the light bulb, are influenced by the electric field. When the switch closes and the electrons are subjected to the electric field, all electrons in the circuit start flowing at seemingly the same time. Those charges nearest the light bulb will take one step through the circuit and start transforming energy from electrical to light (or heat).
History of invention of electricity
Long before any knowledge of electricity existed, people were aware of shocks from electric fish. Ancient Egyptian texts dating from 2750 BCE referred to these fish as the "Thunderer of the Nile", and described them as the "protectors" of all other fish. Electric fish were again reported millennia later by ancient Greek, Roman and Arabic naturalists and physicians. Several ancient writers, such as Pliny the Elder and Scribonius Largus, attested to the numbing effect of electric shocks delivered by catfish and electric rays, and knew that such shocks could travel along conducting objects. Patients suffering from ailments such as gout or headache were directed to touch electric fish in the hope that the powerful jolt might cure them. Possibly the earliest and nearest approach to the discovery of the identity of lightning, and electricity from any other source, is to be attributed to the Arabs, who before the 15th century had the Arabic word for lightning (raad) applied to the electric Ray.
Ancient cultures around the Mediterranean knew that certain objects, such as rods of amber, could be rubbed with cat's fur to attract light objects like feathers. Thales of Miletus made a series of observations on static electricity around 600 BCE, from which he believed that friction rendered amber magnetic, in contrast to minerals such as magnetite, which needed no rubbing.Thales was incorrect in believing the attraction was due to a magnetic effect, but later science would prove a link between magnetism and electricity. According to a controversial theory, the Parthians may have had knowledge of electroplating, based on the 1936 discovery of the Baghdad Battery, which resembles a galvanic cell, though it is uncertain whether the artifact was electrical in nature.

Benjamin Franklin conducted extensive research on electricity in the 18th century, as documented by Joseph Priestley (1767) History and Present Status of Electricity, with whom Franklin carried on extended correspondence.
Electricity would remain little more than an intellectual curiosity for millennia until 1600, when the English scientist William Gilbert made a careful study of electricity and magnetism, distinguishing the lodestone effect from static electricity produced by rubbing amber.He coined the New Latin word electricus ("of amber" or "like amber", from ἤλεκτρον, elektron, the Greek word for "amber") to refer to the property of attracting small objects after being rubbed. This association gave rise to the English words "electric" and "electricity", which made their first appearance in print in Thomas Browne's Pseudodoxia Epidemica of 1646.
Further work was conducted by Otto von Guericke, Robert Boyle, Stephen Gray and C. F. du Fay. In the 18th century, Benjamin Franklin conducted extensive research in electricity, selling his possessions to fund his work. In June 1752 he is reputed to have attached a metal key to the bottom of a dampened kite string and flown the kite in a storm-threatened sky. A succession of sparks jumping from the key to the back of his hand showed that lightning was indeed electrical in nature. He also explained the apparently paradoxical behavior of the Leyden jar as a device for storing large amounts of electrical charge in terms of electricity consisting of both positive and negative charges.
In 1791, Luigi Galvani published his discovery of bioelectromagnetics, demonstrating that electricity was the medium by which neurons passed signals to the muscles. Alessandro Volta's battery, or voltaic pile, of 1800, made from alternating layers of zinc and copper, provided scientists with a more reliable source of electrical energy than the electrostatic machines previously used.The recognition of electromagnetism, the unity of electric and magnetic phenomena, is due to Hans Christian Ørsted and André-Marie Ampère in 1819-1820; Michael Faraday invented the electric motor in 1821, and Georg Ohm mathematically analysed the electrical circuit in 1827.Electricity and magnetism (and light) were definitively linked by James Clerk Maxwell, in particular in his "On Physical Lines of Force" in 1861 and 1862.
While the early 19th century had seen rapid progress in electrical science, the late 19th century would see the greatest progress in electrical engineering. Through such people as Alexander Graham Bell, Ottó Bláthy, Thomas Edison, Galileo Ferraris, Oliver Heaviside, Ányos Jedlik, William Thomson, 1st Baron Kelvin, Charles Algernon Parsons, Werner von Siemens, Joseph Swan, Reginald Fessenden, Nikola Tesla and George Westinghouse, electricity turned from a scientific curiosity into an essential tool for modern life, becoming a driving force of the Second Industrial Revolution.
In 1887, Heinrich Hertz:(843–844) discovered that electrodes illuminated with ultraviolet light create electric sparks more easily. In 1905 Albert Einstein published a paper that explained experimental data from the photoelectric effect as being the result of light energy being carried in discrete quantized packets, energising electrons. This discovery led to the quantum revolution. Einstein was awarded the Nobel Prize in Physics in 1921 for "his discovery of the law of the photoelectric effect".The photoelectric effect is also employed in photocells such as can be found in solar panels and this is frequently used to make electricity commercially.
The first solid-state device was the "cat's-whisker detector" first used in the 1900s in radio receivers. A whisker-like wire is placed lightly in contact with a solid crystal (such as a germanium crystal) in order to detect a radio signal by the contact junction effect. In a solid-state component, the current is confined to solid elements and compounds engineered specifically to switch and amplify it. Current flow can be understood in two forms: as negatively charged electrons, and as positively charged electron deficiencies called holes. These charges and holes are understood in terms of quantum physics. The building material is most often a crystalline semiconductor.
The solid-state device came into its own with the invention of the transistor in 1947. Common solid-state devices include transistors, microprocessor chips, and RAM. A specialized type of RAM called flash RAM is used in USB flash drives and more recently, solid-state drives to replace mechanically rotating magnetic disc hard disk drives. Solid state devices became prevalent in the 1950s and the 1960s, during the transition from vacuum tubes to semiconductor diodes, transistors, integrated circuit (IC) and the light-emitting diode (LED)...... (From Wikipedia)